OcuHealth has developed SMrT (Sustained Microemulsion release Technology) technology to allow vastly greater delivery of drugs to the front of the eye, over an extended period, while bolstering the tear film.
- Sustained, substantial drug release over greater than 12 hours
- Substantially increased drug bioavailability
- Delivers both hydrophobic and hydrophilic molecules
- Monoclonal antibody and small molecules
- Extended release of lipids into tear film reducing evaporation and boosting tear properties
- Lower dosing, improved compliance
- Improved safety and side effect profile
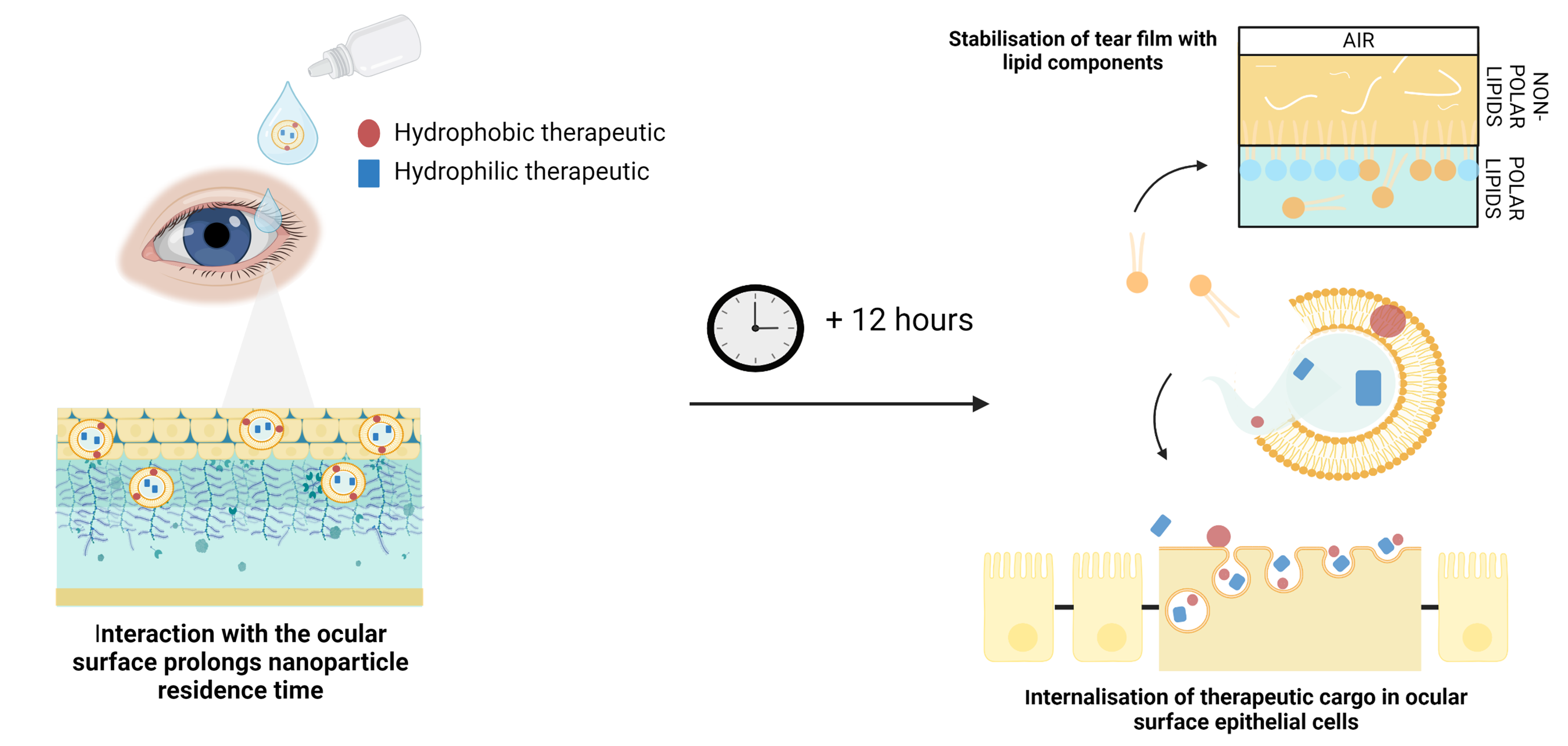
Eye drops are the preferred form of ocular delivery to the front of the eye, with over 90% of ophthalmic drugs available in drop formulations. However, drops are quickly (1-2 minutes) washed away by the eye, due to reflex blinking (5-7 per minute) and reflex tearing, resulting in limited absorption as the drug is not in contact with the ocular surface for very long. Combined with other physiological barriers in the eye, conventional drops result in the loss of 95-99% of drug. This causes the need for frequent administration and higher dosing than the required therapeutic levels to get sufficient drug absorbed in a short window.
There are strong links between compliance and outcomes in the treatment of ophthalmic conditions. The need for frequent administration results in poor compliance in eyedrop products and dry eye patients, for example, can often require up to 16 drops a day.
High dosing means that drug side effects are amplified, and drug is likely to spill over to non-targeted tissues, often with undesired consequences and complications. Side effects are often serious, with issues such as increased intraocular pressure and a higher risk of glaucoma providing clear reasons to discontinue therapy and move to alternate drugs.
High dosing also means that the PK profile is not ideal with large variations in the amount of drug available to act therapeutically in the eye.
Existing eye drop formulations, due to their composition, often have a deleterious effect on the eye, causing unwanted deterioration in the tear film and damage to the ocular surface.
Injectable and depot-based formulations have been developed to try to deal with these issues, but the need for physician administration, patient dislike, risk of dose dumping and other issues mean that these remain niche solutions. Even recently approved nanotechnology-based eye drops are rudimentary, being designed to have one function, i.e. faster penetration or increased drug concentration, and they have little impact on eye health outside of that which the drug payload provides.
An ideal matrix for eyedrop delivery of drugs would have:
- Exquisite targeting of only the ocular tissues where drug is desired
- Long residence time of the drug at the ocular surface, allowing long term absorption
- Reduced dosing frequency – ideally once daily
- Ingredients which actively improve the tear film and ocular surface
OcuHealth’s, patented technology provides a dual solution:
Immediate uptake – allowing much greater absorption of drug into only the cells targeted on the ocular surface by our bespoke nanoparticles and so increasing the speed and quantity of drug uptake.
Their unique combination of surface properties enable OcuHealth’s nanoparticles to interact with, and navigate components of, the ocular surface (e.g. the mucosal layers and surfaces, as well as the cell membrane) in such a way as to significantly increase the level of drug absorption compared to typical drug suspension.
Sustained Release – Rapidly fusing large numbers of lipid nanoparticles to the ocular surface creates and environment whereby the carriers can then release their cargo over an extended period of time. The drug cargo is internalised in the cells, while the lipid components (ALA, Phospholipid and Cholesterol) are released into the tear film, buffering its lipid layer and reducing tear evaporation.
Proof of Concept
Preclinical rabbit models of have been treated with a proprietary nanoparticular liposome formulation of dexamethasone, a potent steroid extensively used for ocular surface inflammatory conditions.
Key outcomes:
- In-vitro drug release maximum at 8h.
- Significant absorption at 12 hour time point = c90% T0
- Negligible ‘spillover’ from local absorption to systemic
- All safety endpoints met
Preclinical mouse safety study
Mice were treated with two nanoformulations alone, or with a cargo of dexamethasone, ciclosporin or novel anti-inflammatory drug. All known drugs were compared to standard of care competitors.
Key outcomes:
- Eyedrops were safe and well-tolerated
- Nanoformulations exhibited a similar safety profile to known competitor drugs
- All safety endpoints met


